
Particles which are smaller than an atom usually referring to protons, neutrons and electrons. Both Democritus and Dalton instructed that matter is made up of ….The of a component is the whole variety of protons and neutrons in the of the atom.Philosophers formulated explanations about the nature of matter primarily based on their own experiences.Grodski Lecture, Grodski evaluate video of the entrance aspect of the worksheet above.
Atomic structure review worksheet pdf#
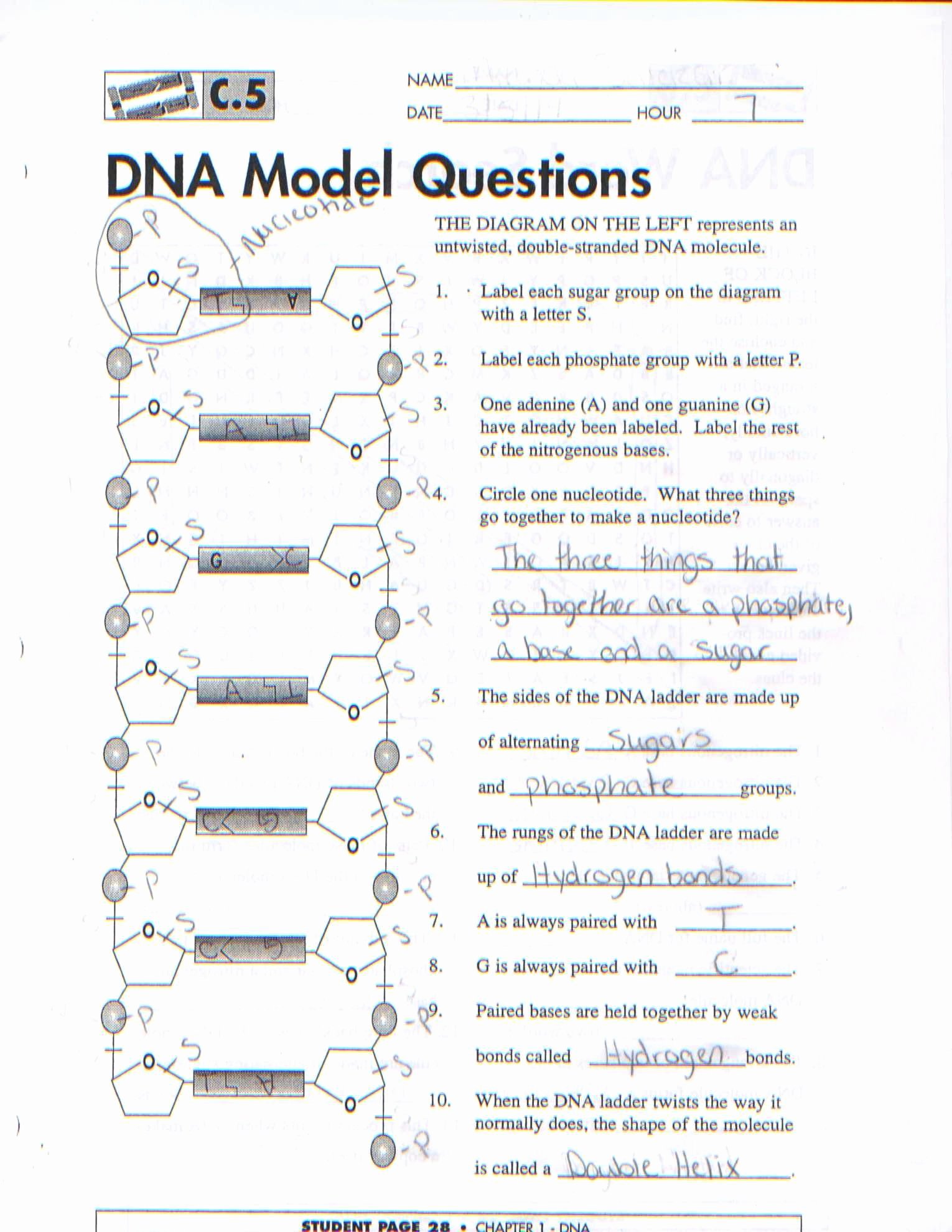
Valence Electrons from Multiple Principle Energy Levels – Electrons are misplaced by metals due to relatively low IE but electrons lost by d – …Ītom of the identical component with the same Z# (# & protons) … Isotope and Ions Practice Worksheet … The similar reply could additionally be used greater than once. The atomic buildings of those hydrogen isotopes are illustrated below. Atomic Structure Worksheet What kind of charge does a proton have. The atomic mass of an element is the common mass of an element’s naturally occurring atom, or. And finally, the charge of an atom is determined by the number of protons and electrons in the atom.Atomic Structure Review Worksheet. The mass number of an atom is found by adding together the number of protons and neutrons. The number of electrons will determine how atoms interact with one another and decide if the atom as a whole is positive, negative, or neutral. The charge of an atom is calculated based on the difference between the number of protons in the nucleus and the number of electrons orbiting the nucleus.Īn atom is made up of protons, electrons, and neutrons. The periodic table arranges atoms in increasing atomic number. Atoms have an atomic number, which is the number of protons in the nucleus.
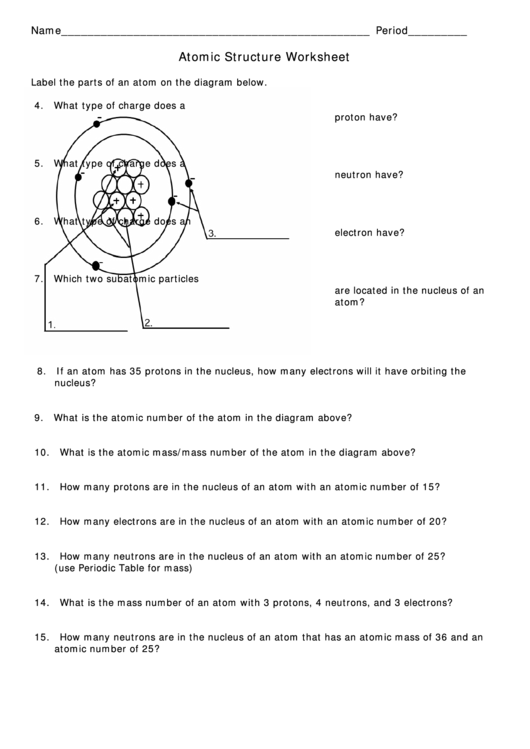
Now that we have talked about the different parts of the atom, let’s summarize a few properties of atoms. Water is made up of two hydrogen atoms and one oxygen atom. Atoms can share electrons to form molecules, which are particles made up of many atoms. The electrons determine how atoms interact with each other. With fewer electrons than protons, the atom will have a positive charge. If the atom has more electrons than protons, its charge will be negative. If the number of electrons is equal to the number of protons, the overall electric charge of the atom is neutral. The electrons are attracted to the positive nucleus, but they can escape their orbit by an outside force.Ītoms have a certain number of electrons orbiting the nucleus. Orbiting the atom’s positively charged center are particles with a negative charge called electrons. This gives us names like Carbon-12 or Carbon-14, which are types of carbon atoms used in carbon dating! To find the mass number of an element you add the number of protons and neutrons together, so protons + neutrons = mass number. Usually, a stable atom has an equal number of neutrons and protons, but there are exceptions. In general, an atom will have a specific number of neutrons in the nucleus, meaning the atom won’t lose or gain any neutrons for a very long time.
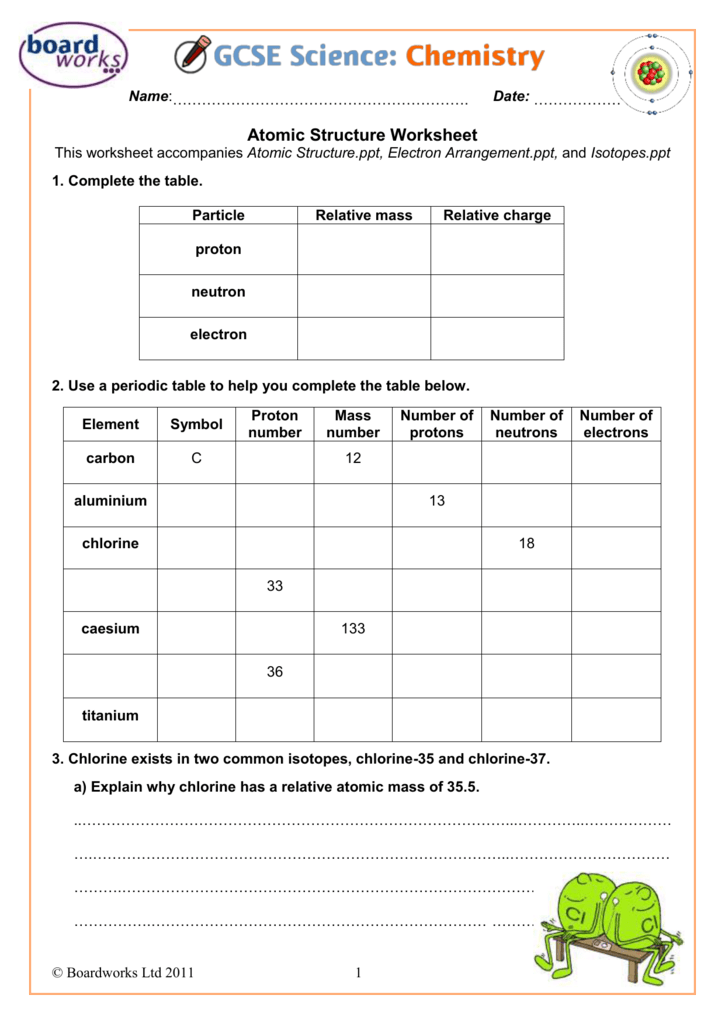
For example, hydrogen is an element with one proton in the nucleus and carbon is an element with 6 protons. A group of atoms that all have the exact same number of protons is called an element. Protons are very small, positively-charged particles, and neutrons are neutral particles that have no charge.Ītoms can have just one proton or they can have multiple. What is an atom?Īt the very center of an atom is the nucleus, which is made up of small particles called protons and neutrons. Atoms are the building blocks of the universe they make up everything you see around you.


 0 kommentar(er)
0 kommentar(er)
