

Landolt-Bornstein: Group II: Atomic and Molecular Physics Volume 7: Structure Data of Free Polyatomic Molecules. "Microwave Spectrum of Methyl Nitrate." Journal of Chemical Physics. NO3- Molecular Geometry / Shape and B n3- lewis sructure molecular geometry. What is the molecular geometry of a compound that has three atoms bonded to a central atom along with three lone pairs of electrons on the. Question: Draw the Lewis structure of NO3 and determine the formal charges of each oxygen and the nitrogen. Please address comments about this page to W. N3- lewis structure molecular geometry Web2 days ago To be very precise. Youll get a detailed solution from a subject matter expert that helps you learn core concepts. NIST does not necessarily endorse the views expressed, or concur with the facts presented on these sites.įurther, NIST does not endorse any commercial products that may be mentioned on these sites. Let me explain this in detail with the help of NO3- lewis structure and its 3D geometry. There may be other web sites that are more appropriate for your purpose. NO3- (or Nitrate ion) is a NONPOLAR ion because all the three bonds (one NO and two N-O bonds) are equidistant and NO3- has symmetrical geometry.

No inferences should be drawn on account of other sites being referenced, or not, from this page.
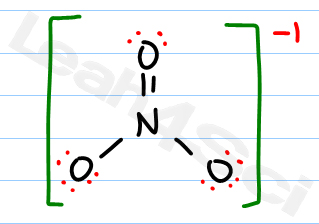
We have provided these links to other web sites because they may have information that would be of interest to you. Internal coordinates (distances (r) in Å) (angles (a) in degrees) (dihedrals (d) in degrees)Įxamples: C-C single bond, C=C, double bond, C#C triple bond, C:C aromatic bondīy selecting the following links, you may be leaving NIST webspace. See section I.F.4 to change rotational constant units A Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to the lone pair on the nitrogen atom.Listing of experimental geometry data for CH 3NO 3 (Methyl nitrate) Rotational Constants (cm -1) This pair exerts repulsive forces on the bonding pairs of electrons. The shape is distorted because of the lone pairs of electrons. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. There are three single bonds and one lone pair of electrons in the NH3 molecule. Thus, Ammonia or NH3 has sp3 hybridization. When it shares the electrons with Hydrogen atoms, one s-orbital and three p-orbitals hybridize and overlap with s orbitals of a Hydrogen atom to form sp3 hybridization. The Nitrogen atom has the electronic configuration of 1s2 2s2 2px1 2py1 2pz1. All the Hydrogen atoms are arranged symmetrically around the Nitrogen atom which forms the base, and the two nonbonding electrons form the tip which makes the molecular geometry of NH3 trigonal pyramidal.
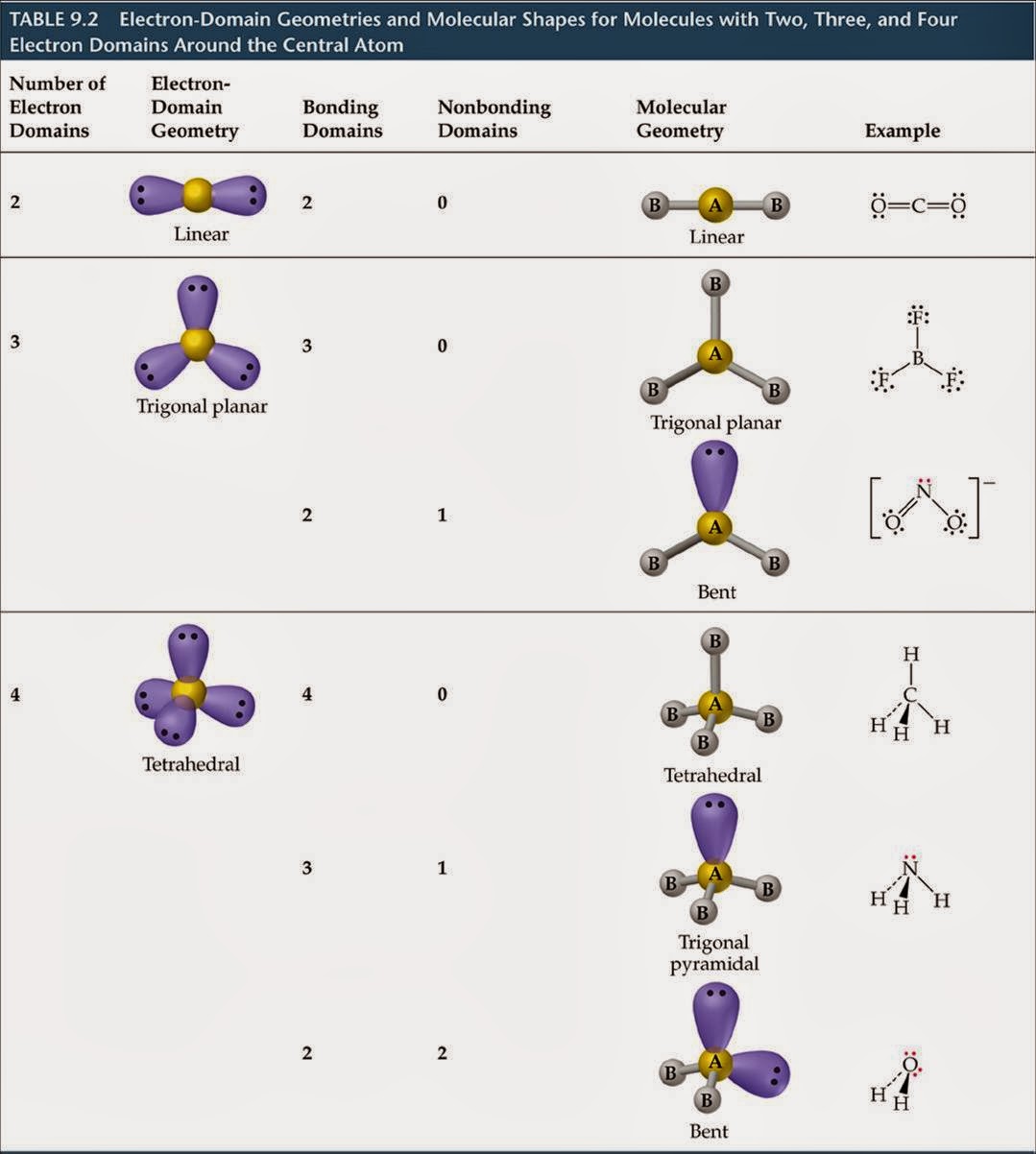
NH3 Molecular GeometryĪmmonia has a tetrahedral molecular geometry. Answer: Theres so much things to consider in the construction of this. Thus there are three single bonds formed between Nitrogen and Hydrogen atoms, and there is one pair of nonbonding electrons on the nitrogen atom. Question: Answer the following questions about the hybrid orbitals on the nitrogen atom in the NO2 and NO3 molecular ions. Nitrogen will share three of its valence electrons for forming a stable structure. Place all the Hydrogen atoms around the Nitrogen atom and the valence electrons of both the atoms like this.Įach Hydrogen atom only needs one electron to become stable, as it is an exception to the octet rule. Hydrogen atoms never take the central position, so we will place the Nitrogen atom in the center. Now that we know the valence electrons for the molecule, we can predict its Lewis structure. Here is the step-by-step procedure to understand the Lewis structure of NH3. The electrons that form bonds are called bonding pair of electrons, whereas the ones that do not form any bonds are called nonbonding pairs of electrons or lone pairs of electrons.ĭots are used to show the valence electrons, whereas the lines represent bonds in the structure. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such properties with ease. Hydrogen – 1 electron, but as there are 3 Hydrogen atoms we will multiply it by 3, there are three valence electrons of all Hydrogen atoms.Īmmonia or NH3 has a total of 8 valence electrons. To get the total number of valence electrons, we will add up the valence electrons for both these atoms. In contrast, Hydrogen is a group 1 element and only has 1 valence electron in its outer shell. Nitrogen is a group 15 element and has five electrons in its outer shell. NH3 Bond angles Valence electrons of NH3 ( Ammonia ).


 0 kommentar(er)
0 kommentar(er)
